2-methylundecanal chanel no 5 | 2 2-methylundecanal chanel no 5 • Burdock, George A., Fenorali, Giovanni. Fenorali’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, 2004. ISBN See more
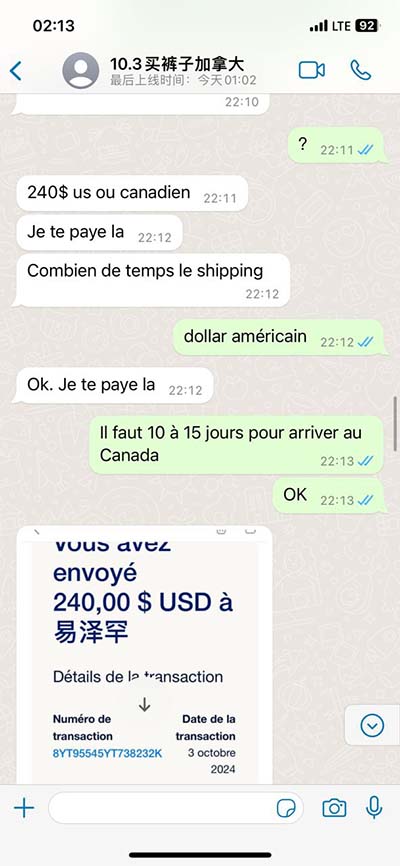
For example, if you jet off to Europe for a month and charge $5,000 on a card that carries a 3% foreign transaction fee, you’ll have to pay an extra $150 when the bill comes due. What’s more,.
0 · 2
Some people feel strongly that words like 'historic' and 'historical' should be preceded by 'an', not 'a'. We recommend using whichever article suits your own pronunciation. Just .
2-Methylundecanal is used widely as a fragrance element in soaps and detergents as well as in the perfume industry to give conifer notes, fir in particular, but is also used in fantasy compositions. This aldehyde was one of the first synthetics to be used in a prestigious perfume, namely Chanel No. 5. See more
2-Methylundecanal is an organic compound that is found naturally in kumquat peel oil. This compound smells herbaceous, orange, and ambergris-like. At high dilution it has a flavor similar to honey and nuts. It is a . See more2-Methylundecanal contains one asymmetric carbon atom.The enantiomers can be synthesized with high enantiomeric . See moreThe first synthesis of 2-methylundecanal was recorded by Georges Darzens in 1904 from methyl nonyl ketone and ethyl chloroacetate. This method of synthesis can be used to produce a variety of aldehydes and became known as the Darzens reaction See more
• Molecule of the Month: Chanel No 5 and 2-methylundecanal See more
• Burdock, George A., Fenorali, Giovanni. Fenorali’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, 2004. ISBN See moreSo Chanel No.5 wasn't the first to use synthetics, but it was one of the first to make major use .2-Methylundecanal is used widely as a fragrance element in soaps and detergents as well as in the perfume industry to give conifer notes, fir in particular, but is also used in fantasy compositions. [6] This aldehyde was one of the first synthetics to be used in a prestigious perfume, namely Chanel No. 5. [8]

So Chanel No.5 wasn't the first to use synthetics, but it was one of the first to make major use of aldehydes. In 1912, the perfumer Robert Bienaimé first used 2-methylundecanal in the successful perfume Quelques Fleurs for Houbigant. Since the 1880s, man-made molecules have increasingly been used, and of these, the medium-sized molecule that has become famous from its association with Chanel Number 5 is a 12-carbon aldehyde, 2-methylundecanal.
2-METHYLUNDECANAL The smell of Chanel No.5 Simon Cotton Uppingham School, Rutland, UK Molecule of the Month November 2008 Also available: JMol versions.
The characteristic fragrance of Chanel No. 5, one of the world’s most well-known perfumes, is due almost wholly to 2-Methylundecanal, a compound found naturally in kumquats. The compound exists in two enantiomeric forms (Figure 1), although professional performers note that the two enantiomers possess nearly identical scent profiles. The aldehyde used in Chanel No. 5 is supposedly C12H24O. On the one hand, they are similar in that they consist solely of carbon, hydrogen, and one oxygen atom. However, I would say that they are not that similar in that the number .The characteristic fragrance, Chanel No. 5, one of the world’s most well-known perfumes, is due almost wholly to 2-Methylundecanal, a compound found naturally in kumquats. The compound exists in two enantiomeric forms (Figure 1 and then they show us a picture of 2-Methylundecanal.An example is 2-methylundecanal which is the typical odor component of Chanel No. 5. [6] Decanal, whose sweet, flowery odor reminiscents of orange peels, is used, among other things, as a flavoring agent in the food industry and as a perfume in the perfume industry.
This is the Molecule of the Month entry for November 2008 about 2-methylundecanal. It is a pdf archive version of the HTML webpage.
This was achieved by several natural and synthetic ingredients in Chanel No. 5's formula, the most well known being an aldehyde accord that included 12-carbon aldehyde 2-methylundecanal (a molecule which appears in the skin of kumquats).
2-Methylundecanal is used widely as a fragrance element in soaps and detergents as well as in the perfume industry to give conifer notes, fir in particular, but is also used in fantasy compositions. [6] This aldehyde was one of the first synthetics to be used in a prestigious perfume, namely Chanel No. 5. [8]So Chanel No.5 wasn't the first to use synthetics, but it was one of the first to make major use of aldehydes. In 1912, the perfumer Robert Bienaimé first used 2-methylundecanal in the successful perfume Quelques Fleurs for Houbigant. Since the 1880s, man-made molecules have increasingly been used, and of these, the medium-sized molecule that has become famous from its association with Chanel Number 5 is a 12-carbon aldehyde, 2-methylundecanal.
2-METHYLUNDECANAL The smell of Chanel No.5 Simon Cotton Uppingham School, Rutland, UK Molecule of the Month November 2008 Also available: JMol versions.
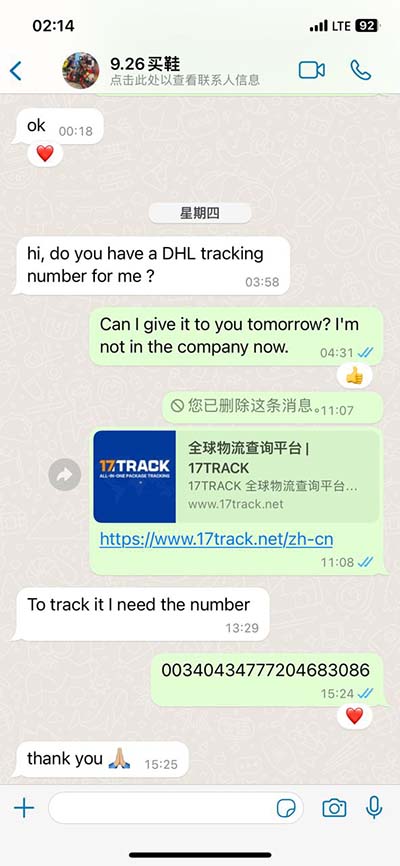
The characteristic fragrance of Chanel No. 5, one of the world’s most well-known perfumes, is due almost wholly to 2-Methylundecanal, a compound found naturally in kumquats. The compound exists in two enantiomeric forms (Figure 1), although professional performers note that the two enantiomers possess nearly identical scent profiles. The aldehyde used in Chanel No. 5 is supposedly C12H24O. On the one hand, they are similar in that they consist solely of carbon, hydrogen, and one oxygen atom. However, I would say that they are not that similar in that the number .The characteristic fragrance, Chanel No. 5, one of the world’s most well-known perfumes, is due almost wholly to 2-Methylundecanal, a compound found naturally in kumquats. The compound exists in two enantiomeric forms (Figure 1 and then they show us a picture of 2-Methylundecanal.An example is 2-methylundecanal which is the typical odor component of Chanel No. 5. [6] Decanal, whose sweet, flowery odor reminiscents of orange peels, is used, among other things, as a flavoring agent in the food industry and as a perfume in the perfume industry.
This is the Molecule of the Month entry for November 2008 about 2-methylundecanal. It is a pdf archive version of the HTML webpage.
The scene with the zodiac love-making positions; the Greek zodiac later plays a role in deciphering the true purpose of Anaconda Malt Liquor. Black Dynamite swears on the ghost of Abraham Lincoln, who helps him to defeat Richard Nixon. That's what Nixon gets for using John Wilkes Booth's derringer.
2-methylundecanal chanel no 5|2